
Periodic table of the elements, showing the atomic species in which
Here we report the precise determination of the 3D coordinates and chemical species of 23,196 atoms in a single 8.4-nm Fe 0.28 Pt 0.72 nanoparticle using atomic electron tomography (AET) 1. FePt.
FileSchematicky atom.png Wikimedia Commons
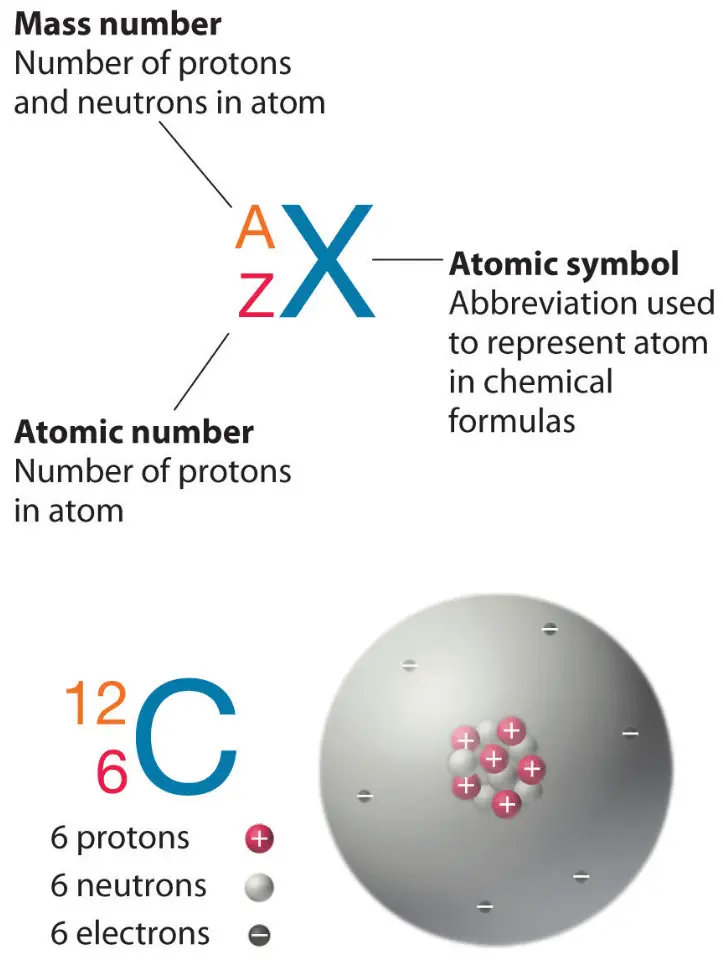
The total mass of neutrons, protons, and electrons found in an atom determines its mass number or atomic number. There are several atomic species centred on this. They are known as isotopes, isotones, isobars, and isoelectronic.

The AOs 2 O 6 lattice. The atomic species are maker Download
First of all, identify the given species whether an atom or ion or molecule. If given species is an atom, its total number of electrons is equal to its atomic number. For example, Argon(Ar) atom has atomic number 18 and a total electron of 18. If the given species is a positively charged ion, then the total number of electron=Atomic number.

SOLVEDIn βdecay, an electron comes out from an atom. The electron
(Dated: December 10, 2019) We report the preparation of a heteronuclear two-atom system of 87Rb and 85Rb in the ground state in an optical tweezer. Dual-species Raman sideband cooling is applied to the two initially separated atoms to eliminate the crosstalk and a 3D ground-state probability of 0.91(5) for 87Rb and 0.91(10) for 85Rb are obtained.
Periodic Variations in Element Properties General Chemistry
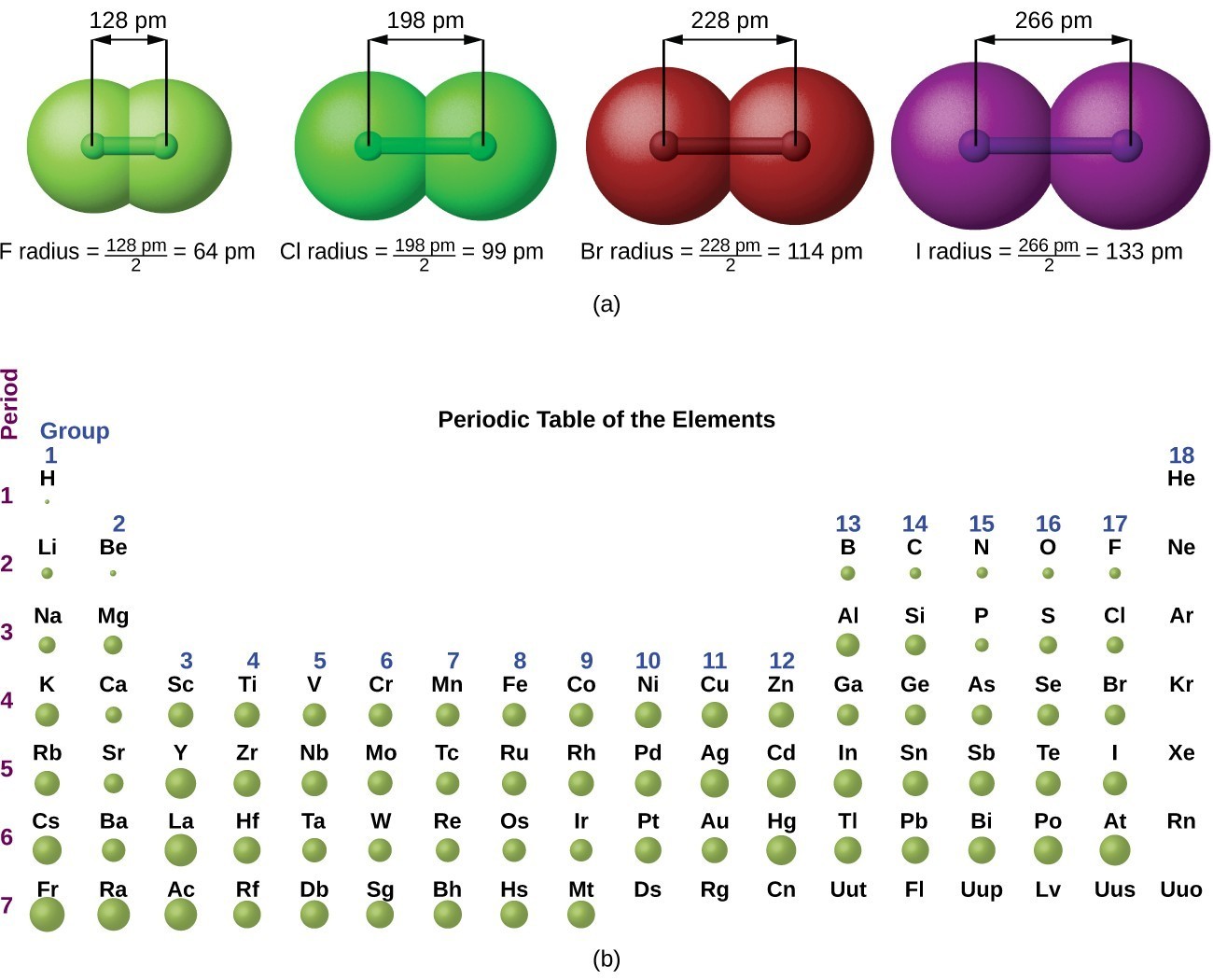
Atomic radii are often measured in angstroms (Å), a non-SI unit: 1 Å = 1 × 10−10 m = 100 pm. Figure 7.3.2 7.3. 2: Definitions of the Atomic Radius. (a) The covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as Cl 2.

(Color online) Proposed mechanism (a) creation of atomic species, (b
The atomic number or mass number of an atom is based on the number of electrons, protons, and neutrons present in them. Based on this, there are different atomic species (isotopes, isobars, isotones, isoelectronic). Atomic Number and Mass Number The number of protons in an atom represents its atomic number. It is denoted by the letter Z.
Iridium Protons Neutrons Electrons Electron Configuration
Fractional Conversion (Interactive) Limiting Reagent (Interactive) Reaction Stoichiometry (Interactive) Three Methods for Reactive MEB Problems. Atomic Species Balances. Extent of Reaction for Material Balances. Molecular Species Balances. Two Reactions in a Two-Phase Reactor. Percent Excess Air.
Example Atomic Species Balance (pt 11) YouTube
The simplest conceivable molecule would be made of two protons and one electron, namely \ (\ce {H2^ {+}}\). This species actually has a transient existence in electrical discharges through hydrogen gas and has been detected by mass spectrometry and it also has been detected in outer space. The Schrödinger equation for \ (\ce {H2^ {+}}\) can be.

Chemical Species Explained Lecture 1Atom and Ion YouTube
21 First, I would like to quote sentences from a book introducing elements and atoms: An element is a fundamental (pure) form of matter that cannot be broken down to a simpler form. Elements are made up of particles called atoms. An atom is the smallest unit of any element [.]

Atomic Species YouTube
The APFIM provided essentially a one-dimensional series of atomic species as the sample was 'eroded' one atomic layer at a time. Continued improvements and new configurations of the hardware through to the mid-1990s resulted in position-sensitive ion detection, in combination with full-spectrum time-of-flight mass spectrometry (Cerezo et al.

Bohr's Model for Hydrogen Atom Postulates, Merits, Limitations
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities. The set of these entities can explore the same set of molecular energy levels on a characteristic or delineated time scale.

Structure Of Atom 01 Atomic Number , Atomic Mass and Atomic Species
Figure 8.2.2 8.2. 2: Definitions of the Atomic Radius. (a) The covalent atomic radius, rcov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as Cl 2. (b) The metallic atomic radius, rmet, is half the distance between the nuclei of two adjacent atoms in a pure solid metal, such as.
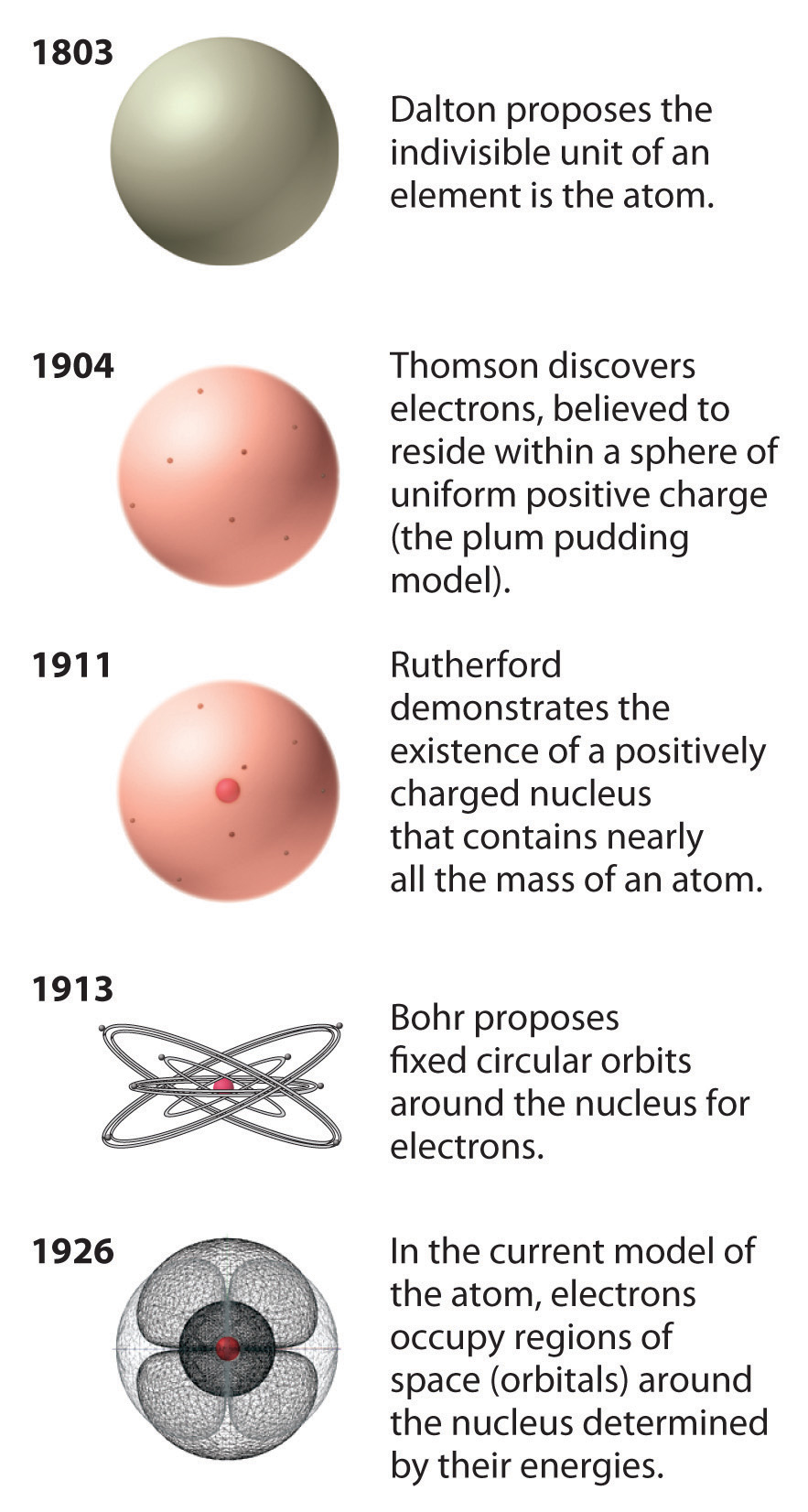
Chapter 1.5 The Atom Chemistry LibreTexts
Synopsis Quantum Computing Arrays Made of Two Types of Atom February 24, 2022 • Physics 15, s20 Two research teams have created arrays containing two different neutral atoms, a promising platform for quantum computing. C. Sheng et al. [ 1]

4 nm thick sections from atom maps showing distribution of atomic
In the field of cold atom inertial sensors, we present and analyze innovative configurations for improving their measurement range and sensitivity, especially attracting for onboard applications. These configurations rely on multi-species atom interferometry, involving the simultaneous manipulation of different atomic species in a unique instrument to deduce inertial measurements. Using a dual.
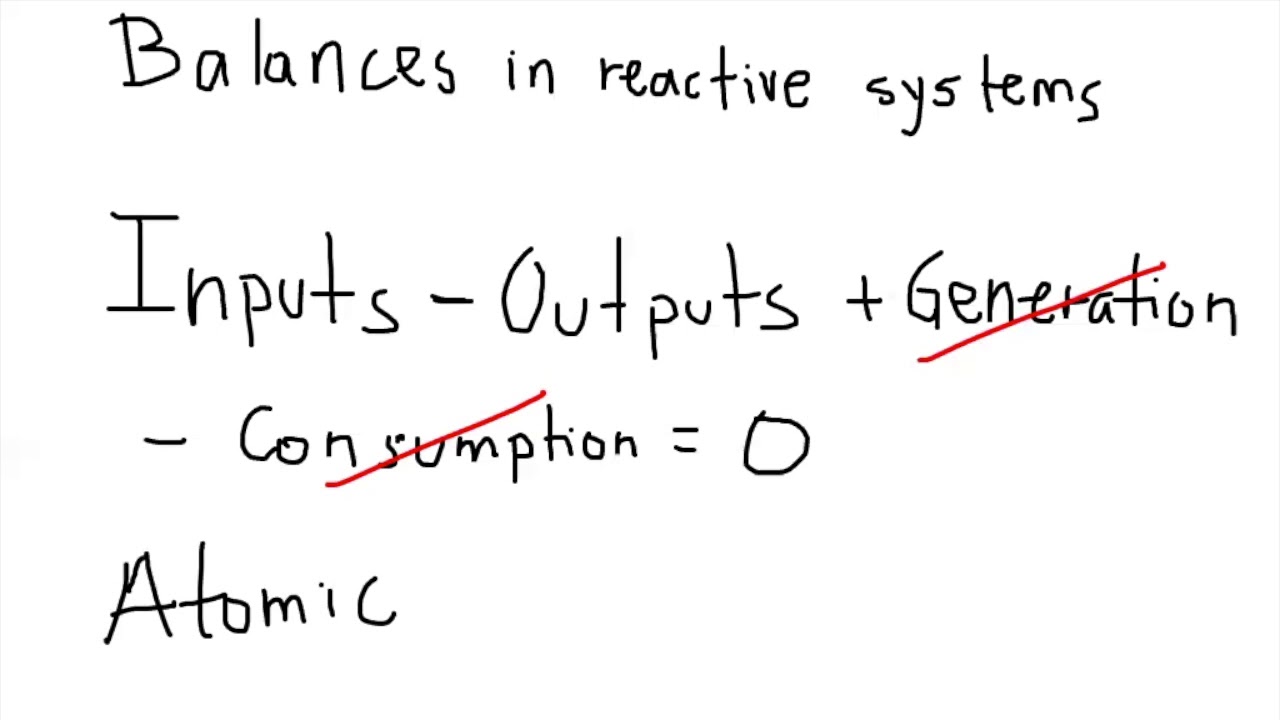
3.2 Balance for atomic species YouTube
9 Citations 1 Altmetric Metrics Abstract The redox states of oxygen species on the surface of TiO 2 can be altered by electron tunneling by varying the applied bias voltage of an atomic force.

Best of Last Week Creating Rydberg polarons, Tesla carrying bacteria
An atom is the smallest unit of ordinary matter that forms a chemical element. Atoms are made of fundamental particles called protons, neutrons, and electrons. Protons and neutrons clump together to form a central nucleus. The electrons move in a cloud-like region around the nucleus. Most atoms are stable.